Loading... Please wait...
Loading... Please wait...Categories
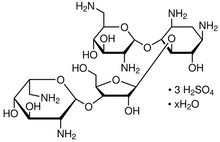
Neomycin Sulfate
| CAS Number: |
1405-10-3
|
| Chemical Formula: |
C23H46N6O13 3H2SO4
|
| Molecular Weight: |
908.9 g/mol
|
Soluble in: Water
Store at: Room Temperature
Neomycin sulfate is an aminoglycoside antibiotic active against most gram negative bacteria. Selective agent for the incorporation of the NPT II (APH3') gene in plant tissue.
Product Specifications:
- Neomycin B: 85%
- Neomycin C: 10%
Free Shipping within the Continental USA
Neomycin Protocol
Background
Neomycin is freely soluble in water (50mg/mL), and sparingly soluble in ethanol and methanol (0.10 mg/mL and 0.23 mg/mL respectively). Neomycin is routinely used to select for cells containing resistance plasmids such as pcDNA3 in common cell lines including AtT-20.
Preparation and storage
For long term storage, we suggest that neomycin sulfate be stored as supplied at -20°C. It should be stable for at least two years. Neomycin (sulfate) is sparingly soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. For biological experiments, we suggest that organic solvent-free aqueous solutions of neomycin sulfate be prepared by directly dissolving the neomycin sulfate compound in aqueous buffers. Do not recommend storing the aqueous solution for more than one day.
Mammalian Cell Culture
Generating stable cell lines in HEK293
1. Prior to transfection, it is recommended that you linearize your pcDNA gene construct. Linearizing will decrease the likelihood of the vector integrating into the genome in a way that disrupts the gene of interest or other elements required for protein expression. Suitable restriction enzymes for linearization are: Bg lII, MfeI, Pvu I, Sca I.
2. Transfect cells in dishes of 6 cm diameter according to the protocol for transient transfection in HEK. About 16 hrs post transfection, exchange the medium to medium supplemented with 0.5 g/L of neomycin.
3. Keep exchanging the medium every day for 7-10 days. This is the time needed for the neomycin to act on the nontransfected cells, which then detach and are washed away during the medium exchange. Once all cells have died in the dish of the negative control (nontransfected), you can proceed with the cloning.
4. Take 4 - 6 (ore more) 96-well plates and fill them with 80 µl of neomycin-supplemented medium. You may pour the medium into a sterile round cell culture dish and use the multichannel pipette and filter tips in this process. Trypsinize the cells (allowing full separation of cells => no clumps!) and resuspend an aliquot of cells in a 15 ml falcon tube. Then make 1 or 2 serial dilutions (e.g. one in ten) and determine the cell concentration with the cytometer. Check your dilutions until you can only count 1-5 cells in the cytometer: the accuracy of your dilutions is very important.
5. Prepare a sufficient amount of a 3cells/20µl (150 cells/ml) dilution. Work fast and don't let the dilutions stand too long or the cells will form clumps. Distribute 20 µl to each well of the plates. Be sure that you constantly shake the cells in the medium (best done when having only few mililiters in a 50 ml falcon tube), in order to distribute them equally to the wells.
6. Let the cells grow in the wells for about 2 weeks. It is useful, if you already mark the wells containing monoclonal colonies (derived from 1 cell) after a few days. About one week after plating, you can also add 100 µl of fresh neomycin-medium to each well to reestablish high neomycin concentration (it may get broken down with time) and prevent contamination.
7. Once "big" colonies (about 1/6 of the well diameter) are visible, also by eye when viewing the wells from the bottom of the plate, and the color of the medium starts to change (typically after about 2 weeks), colonies can be screened for expression by ELISA. At this stage, expression values typically are between 0.05 and 0.2 µg/ml.
8. Choose the best clones to transfer them to a 24-well plate: First, prepare the wells in the new plate with about 400 µl of medium. Then aspirate the medium from the cells in the old wells and wash them with PBS, add trypsin (few 40 µls) and wait until cells have detached (about 1 minute). Gently resuspend the cells with the pipette. Add 80 µl of medium to the cells and transfer them to the fresh well. Let them grow and determine the protein-expression levels again once they have reached a suitable cell number.
9. Since expression levels depend on the cell number, it is hard to determine the best expressing clone at the level of 24- or 6 well plates. Thus, it is best to grow several candidates up to the 75 cm2 level.
Establishment of cell lines that constitutively express GFP-tagged proteins
1. Clone your protein of interest into one of Clontech’s Living Colors vectors (EGFP, EFYP, ECFP, etc.) These vectors encode kanamycin resistance for selection in bacteria and neomycin (G418) for selection in mammalian cells.
2. Characterize expression of the fusion protein in your cell type of interest using transient transfections. You want to be sure that your fusion protein is full-length and not degraded (can probe with GFP antibody on a Western blot; Roche’s monoclonal anti-GFP works well at 1:1000). If you have an antibody to the endogenous protein you can also compare the level of expression of the fusion protein to the endogenous protein. Many proteins mislocalize when overexpressed. Include coverslips in the dish that can be fixed to check localization by fluorescence microscopy.
3. To set up for the stable cell line selection, split cells and transfect with your construct. The following day, replace the standard media with media containing G418. Over time this will select for cells that have stablyincorporated the GFP plasmid into their genomic DNA. The amount of G418 required to kill cells not expressing the construct will vary from cell line to cell line. We have titrated the amount required for our HeLa cell lines, and this can be a starting point for other cell lines. Initially we use 400 ug/ml G418 in the media when selecting for stable clones. This selection can take anywhere from 1 to 2 weeks. Carefully change the media in the dish every day, taking care not to pipette directly onto the cells. At some point there will be a massive cell death and most of the cells will wash off the bottom of the dish, leaving colonies of stable cells behind.
4. To pick colonies, prepare 24-well dishes with 1 ml media containing G418 in each well. Rinse the dish with PBS and then add warm PBS containing 5% trypsin (1 ml standard trypsin-EDTA plus 19 ml PBS). Colonies can be picked on an inverted light microscope, although an inverted fluorescence microscope allows you to monitor the fluorescence signals in the colonies that you’re picking. Using a Gilson pipette with a sterile yellow tip, lower the tip to the surface of the colony of interest and scrape and suck gently until you’ve pulled it up into the tip. Transfer to a well in the 24-well plate. Repeat with other colonies.
5. In a day or two, when the wells are confluent, rinse with PBS and trypsinize with 100 ul of trypsin-EDTA. Split into one well of a 6-well plate (for passaging) and one well of a 24-well plate that also contains a 13 mm coverslip. These coverslips can then be fixed in a day or two to screen the colonies to decide which should be put down and which are worth keeping (and whether they require further subcloning to reach > 95% homogeneity).
6. All clones that you are interested in keeping should be passaged from the 6-well plate into at least 3 dishes: one for freezing as Passage 0 (in case something goes horribly wrong in the future you can always go back to this one), one for further passaging (if the line is already clonal), and one for subcloning (if required). Seed at clonal density for subcloning (e.g. 10ul from a 1:5 split of the 6-well dish into a 10 cm dish).
7. When you have your clone or subclone of interest, you can then use a lower amount of G418 for maintenance (200 ug/ml for HeLa cells). Keep track of passage numbers (stability of the clones will vary and some maybe thrown out after a few passages). Periodically freeze down samples from early passages so that you can always go back to them.
Comparable Items:
| American Radiolabeled Chem MS | ARCD0722 1 |
| Alfa Aesar | J61499-14 |
| Alfa Aesar | J61499-22 |
| Amresco | E482-20ML |
| Amresco | 0558-25G |
| Amresco | 0558-100G |
| EMD Millipore | 480100-20ML |
| EMD Millipore | 4801-25GM |
| TCI America | F0649-25G |
| US Pharmacopeia | 1458009 |