Loading... Please wait...
Loading... Please wait...Categories
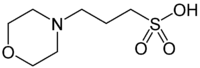
MOPS
(3-(N-morpholino)propanesulfonic acid; 4-Morpholinepropanesulfonic acid)
Purity > 99.5%
| CAS Number: |
1132-61-2
|
| Chemical Formula: |
C7H15NOS
|
| Molecular Weight: |
209.3 g/mol
|
MOPS buffer is used for the electrophoresis of RNA through agarose gels. In cell biology, MOPS can be used for the isolation of plant organelles such as chloroplasts. Useful pH Range 6.5 - 7.9
Free Shipping within the Continental USA
Protocol for the Preparation of 10x MOPS Electrophoresis Buffer
1) Dissolve 41.8 g of MOPS in 700 ml of sterile DPEC treated H2O. Adjust the pH to 7.0 N NaOH.
2) Add 20 ml of DEPC treated 1 M sodium acetate and 20 ml of DEPC treated of 0.5 M EDTA pH 8.0. Adjust the volume of the solution to 1 L with DEPC treated H2O.
3) Sterilize by filtering through a 0.45 um and store protected from sunlight.
The purpose of DEPC (diethylpyrocarbonate) is to eliminate RNAse activity.
Required Reagents
20 mM sodium acetate trihydrate 10 mM EDTA pH 8.0
- 0.2 M MOPS pH 7.0
- 20 mM sodium acetate trihydrate
- 10 mM EDTA pH 8.0
Comparable Items:
| Alfa Aesar | J61843-AP |
| Alfa Aesar | A12914-36 |
| Alfa Aesar | J62261-AK |
| Alfa Aesar | A12914-22 |
| Alfa Aesar | J60328-AE |
| Alfa Aesar | J63369-AK |
| Alfa Aesar | J62261-AP |
| Alfa Aesar | J61843-AK |
| Alfa Aesar | J63369-AE |
| Alfa Aesar | J62476-AE |
| Alfa Aesar | J60922-AK |
| Alfa Aesar | J62368-AK |
| Alfa Aesar | J60097-AK |
| Alfa Aesar | J60922-AE |
| Alfa Aesar | J62476-AK |
| Alfa Aesar | A12914-14 |
| Alfa Aesar | J60328-AK |
| Alfa Aesar | J60097-AP |
| Alfa Aesar | J62368-AE |
| Amresco | 0670-100G |
| Amresco | 0670-250G |
| Amresco | E526-500ML |
| Amresco | 0670-500G |
| Amresco | E526-100ML |
| Amresco | M214-100G |
| G-Biosciences | RC-073 |
| G-Biosciences | RC-072 |
| JT Baker | 4810-06 |
| JT Baker | 4810-02 |
| JT Baker | 4810-04 |
Celebrity Endorsements
-The paper examines a number of buffers developed by Good (often called “Good’s” or “Good” buffers) have been shown to cause metal ion interference as a result of complexation. This can be critical in protein crystallization especially if metals are needed for proper protein folding. A series of tertiary amines, devoid of hydroxy groups or other weak donors on the r, â, or ç carbons, have been developed as “Better” pH buffers which, as a result of steric hindrance, are incapable of forming even weak complexes with metal ions. MOPS, MES and PIPES are the only three of the twenty Good Buffers that were examined that do not show weak coordination. Unfortunately, PIPES can be limited due to solubility in biologically relevant conditions.
Goldber, DA. (1980) Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc. Natl. Acad. Sci. USA. Vol. 77, 5794-5798.
Oldfield, TJ. Ceska, TA. Brady, RL. (1991) A flexible approach to automated protein crystallization. Journal of Applied Crystallography. Vol. 24. 255-260.
-MOPS is used as an example buffer in this paper discussing a set up for automated protein crystallization.