Loading... Please wait...
Loading... Please wait...Categories
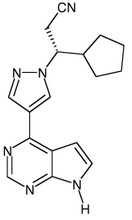
Ruxolitinib, Free Base
Purity > 99.0%
| CAS Number: |
941678-49-5
|
| Chemical Formula: |
C17H18N6
|
| Molecular Weight: |
306.37 g/mol
|
Ruxolitinib is the generic name ruxolitinib is also known as Jakafi. It is sold as ruxolitinib in the United States to be used in the treatment of myelofibrosis. The drug Jakafi was designed by the Incyte Corporation in conjunction with Novartis to treat myelofibrosis. The European Commission and the FDA both approved Jakfi to be used in the treatment of myelofibrosis. In June of 2011, the Incyte Corporation applied to the FDA for FDA approval of ruxolitinib as a myelofibrosis treatment following their results based on two research trials used to determine the efficacy of ruxolitinib in the treatment of myelofibrosis. The results of their trials proved conclusively that ruxolitinib was effective in the treatment of myelofibrosis and the FDA approved the drug ruxolitinib on November of 2011. Actually, the European drug Jakafi used as ruxolitinib in the United States was first approved in the treatment of myelofibrosis in the United States by the FDA before it was approved by the European Commission on August of 2012.
The disease known as myelofibrosis shows up as scar tissue in bones and actually replaces bone marrow. It is a type of cancer of the blood. The cancer that causes myelofibrosis prevents blood from entering the affected bones which dries up the bone marrow and leaves scar tissues. The ensuing symptoms are anemia, fatigue and the extending of the bone cancer to other bodily organs namely the spleen and the liver. Both organs enlarge. The enlargement of the spleen and liver shows up in x-rays which is one way of diagnosing the disease. In the trials held by the Incyte Corporation used x-rays of the spleen and liver to determine if their drug ruxolitinib was effective in the treatment of the myelofibrosis. Their tests showed a reduction in the size of the spleen and liver in patients diagnosed with having myelofibrosis. That result formed the basis of their decision that their drug was effective in the treatment of the disease and was accepted as proof of the drug's efficacy by the FDA. The drug acts as a block to the protein molecule that invades the bone marrow causing the cancerous effects of killing blood cells in the bone marrow and of preventing new blood cells from forming. The rights to the drug in the United States are held by the Incyte Corporation and in Europe the drug is sold and made by Novartis.
Ruxolitinib, free base is for in vitro (not human or animal consumption) use only.
Soluble in DMSO at 28 mg/mL
Ethanol at 15 mg/mL with warming
Very poorly soluble in water; maximum solubility in plain water is estimated to be about 25-50 µM
Buffers, serum, or other additives may increase or decrease the aqueous solubility.
Storage -20 ºC
Free Shipping within the Continental USA