Loading... Please wait...
Loading... Please wait...Categories
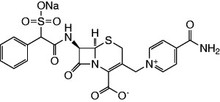
Cefsulodin Sodium Salt
(Cefsulodin (SCE-129); Cefomonil; Pseudocef)
Purity > 97.0 %
| CAS Number: |
52152-93-9
|
| Chemical Formula: |
C22H19N4O8S2Na
|
| Molecular Weight: |
554.53 g/mol
|
Cefsulodin is a third generation cephalasporin antibiotic that inhibits cell wall. It has high activity against Pseudomanas aeruginose with no significant activity against other Gram negative bacteria. Limited activity against Gram positive and anaerobic bacteria.
Background
Cefsulodin Sodium is a third generation cephalosporin antibiotic used as a selective antibiotic in CIN (Cefsulodin, Irgasan, Novobiocin) agar (also called Yersinia Selective Agar) for the selective isolation ofYersinia enterocolitica. Yersinia is oen of the common pathogens identified in water and beverage. Cefsulodin is also used to study the effect of ex- pression, binding, and inhibition of PBP1 and other penicillin-binding proteins (PBPs) on bacterial cell wall mucopeptide synthesis.
Cefsulodin was first synthesized and patented by Takeda Pharmaceutical Company in 1977. In 2002, Takeda stopped production of Cefsulodin. Many years of low-stability cefsulodin production has led to a widespread reduction of laboratory and research usages.
Current attempts (i.e. IDEXX Laboratories, Inc.) of increasing purity and stability of cefsulodin center around recrystallization. Typically the process entails the following: Cefsulodin is dissolved in an organic solvent, sodium, water or any mixture thereof, then subsequently recrystallized through separation of the unwanted fraction.
Recently, improvements have been made to improve cefsulodin stability by the removal of one key impurity in 7-ACA (7-aminocephalosporanic acid, which is a raw material used in the synthesis of cefsulodin). In order to produce high-purity, high-stability cefsulodin, industrial HPLC has been added to purification protocol to remove significant quantities of this impurity and thus produce ultra-pure, ultra-stable, and ultra-potent cefsulodin.
Free Shipping within the Continental USA